
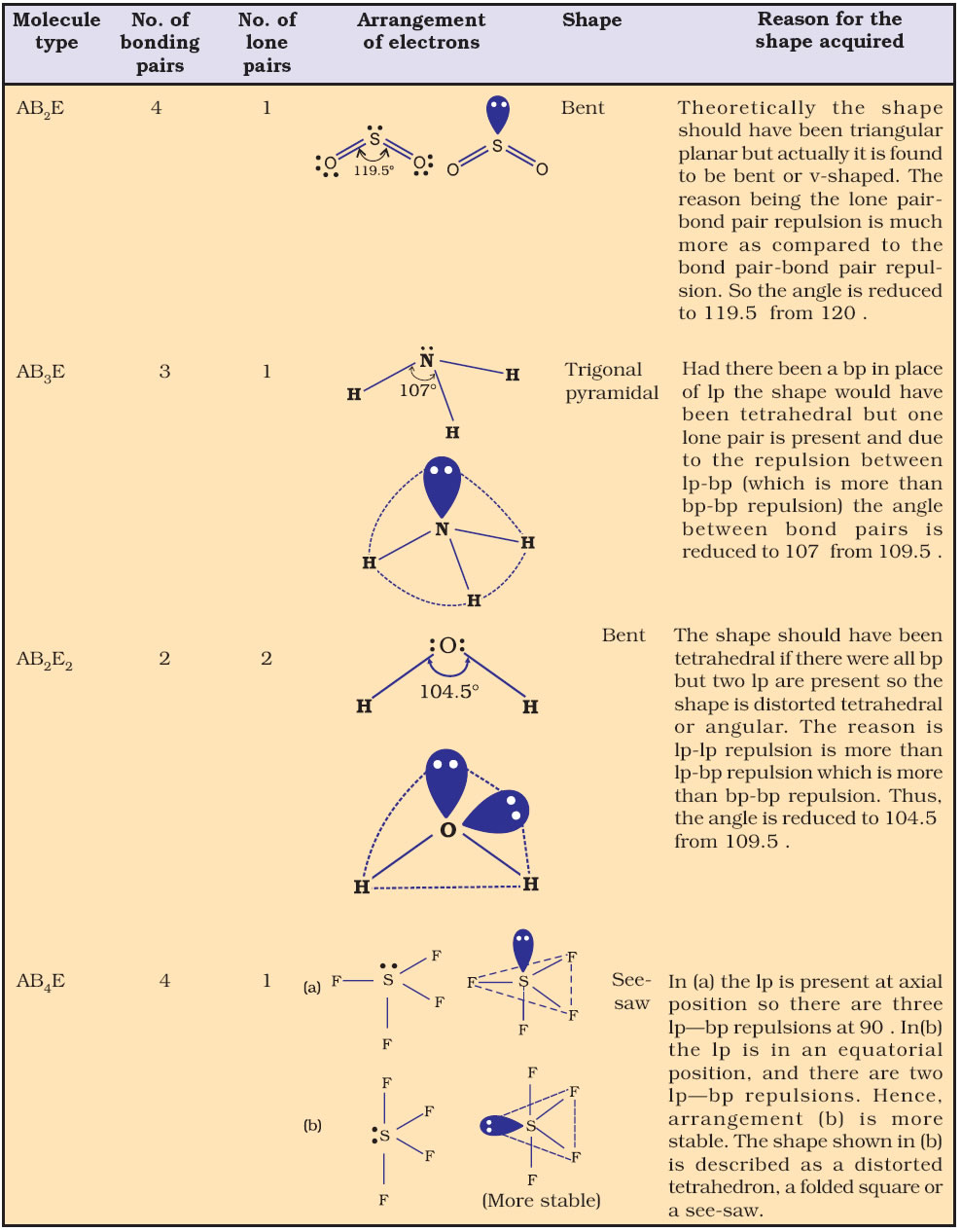
The shape the two bonding pairs take up around the central S atom is NON-LINEAR or BENT. The four electron pairs take up a tetrahedral arrangement to get as far apart as possible, to the bond angles are 109.5 – (2 x 2.5) = 104.5º. H 2S S has 2 bonding pairs S has 2 lone pairs The shape the three bonding pairs take up around the central N atom is PYRAMIDAL. The four electron pairs take up a tetrahedral arrangement to get as far apart as possible, so the bond angles are 109.5 – 2.5 = 107º. NF 3 N has 3 bonding pairs N has 1 lone pair When we determine the bond angles we also need to remember that the repulsion of the lone pairs towards bonding pairs is stronger than the repulsion between bonding pairs, so bond angles can be reduced by 2.5º. The lone pairs behave as invisible forces pushing the bonding pairs into new positions, and in naming the shape we only consider where the bonding pairs have ended up under the influence of these invisible lone pairs.
The electron pairs will still arrange themselves to get as far apart as possible: both the lone pairs and the bonding pairs will do this. Molecules where the central atom has lone pairs as well as bonding pairs Each lies at 90° to the others. The shape is OCTAHEDRAL. There are 6 bonding pairs arranging themselves as far apart as possible. e.g. SF 6 S has 6 bonding pairs S has 0 lone pairs Three lie in a plane, with 120° bond angles and the other two are at 90° to this plane. There are 5 bonding pairs arranging themselves as far apart as possible. This leads to a 109.5° bond angle and a TETRAHEDAL shape.Į.g. PF 5 P has 5 bonding pairs P has 0 lone pairs There are 4 bonding pairs arranging themselves as far away as possible.

CH 4 C has 4 bonding pairs C has 0 lone pairs

This leads to 120° bond angles in a planar molecule. There are 3 bonding pairs arranging themselves as far away as possible.
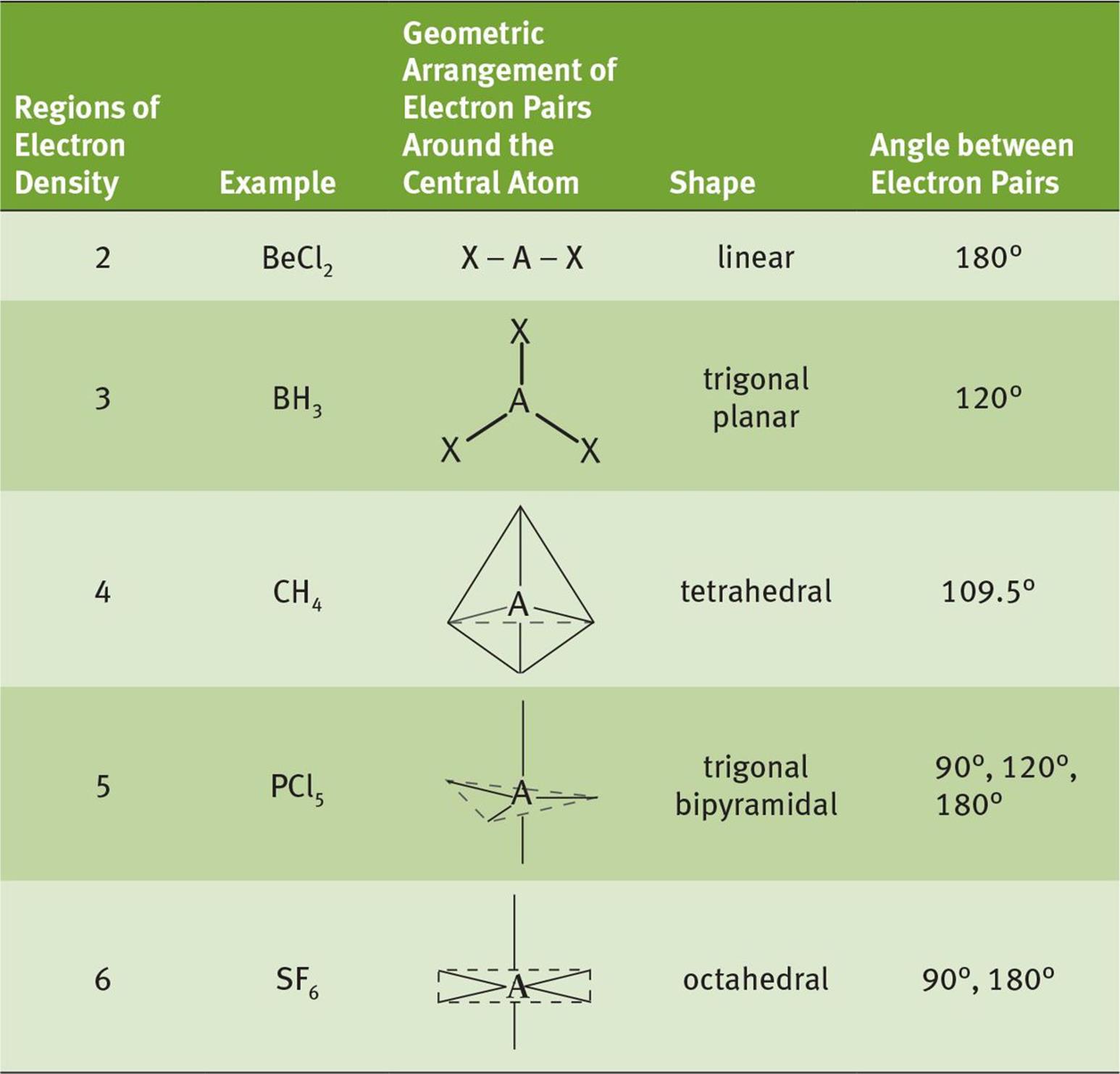
BF 3 B has 3 bonding pairs B has 0 lone pairs The lone pairs on Cl have no effect on the shape. The two bonding pairs repel as far as possible from one another, giving a bond angle of 180°. BeCl 2 Be has 2 bonding pairs Be has 0 lone pairs Molecules with only bonding pairs around the central atom e.g. To understand how these shapes arise, we need to look at specific molecules and in particular to examine the number and type of electron pairs around the central atom. VSEPR Theory states: i) electron pairs repel to get as far apart as possible and ii) lone pairs repel more strongly than bonding pairs.Īs a consequence of this, a lone pair will push bonding pairs closer together, decreasing the angle between them by 2.5º per lone pair.Įach specific shape has a name that reflects its geometry: It is the mutual repulsion between these electron pairs which gives the molecule its shape and bond angles. The outer shell of this central atom will contain electrons which are part of bonding pairs, and may also contain lone pairs. We will consider a simple molecule as consisting of a central atom, around which a number of other atoms are arranged. The shapes of molecules and their bond angles (the angles between the covalent bonds) are not random, but are determined according to a set of principles called Valence Shell Electron Pair Repulsion Theory.


 0 kommentar(er)
0 kommentar(er)
